Note:
The following product information is intended for use by healthcare professionals only.
The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
The only new PD-1 monoclonal antibody that applies the IgG1 subtype with modified Fc domain
安尼可® (PD-1 Monoclonal Antibody, Penpulimab injection)
安尼可® (PD-1 Monoclonal Antibody, Penpulimab Injection) is currently the only differentiated PD-1 monoclonal antibody that applies the IgG1 subtype with modified Fc-nulldomain, which can more effectively enhance immunotherapeutic efficacy and reduce immune-related adverse reactions. It is used in the treatment of major diseases such as lung cancer, lymphoma, nasopharyngeal cancer, liver cancer, and gastric cancer, with improved safety and efficacy as demonstrated in clinical studies.
安尼可® has been approved by the National Medical Products Administration of China (NMPA) for :
1.The first-line treatment of recurrent or metastatic nasopharyngeal cancer (NPC) in combination with chemotherapy;
2.The first-line treatment of locally advanced or metastatic squamous NSCLC;
3.The treatment of relapsed or refractory classical Hodgkin's lymphoma (r/r cHL) after at least second-line systemic therapy;
4.The third-line treatment of metastatic nasopharyngeal cancer chemotherapy.
In addition, the clinical trials of 安尼可® for the treatment of liver cancer and gastric cancer are progressing efficiently.

Second-generation PD-1 monoclonal antibody with an optimized structure and better efficiency and safety
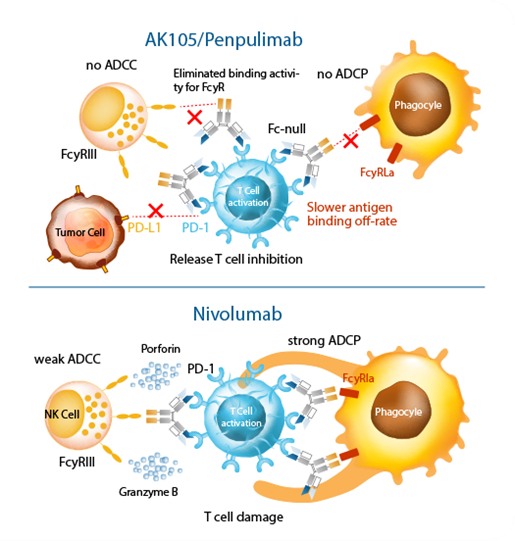
① IgG1 subtype:It has better therapeutic effect with stability more than 100 times that of natural IgG4; it won’t bind with other IgG, and mediates no immune escape; it has lower host protein residue, and reduces fevers and infusion reactions caused by host protein;
② Fc modification:It completely removes ADCC/ADCP/CDC, avoids phagocytosis or killing of immune cells, reduces ADCR, enhances efficacy by reducing IL-8 release, and decreases irAE by reducing IL-6 release;
③ Fab optimization:It binds strongly to PD-1 and dissociates slowly with high affinity and low EC50, effectively activates immune cells with excellent specificity, and causes no adverse reactions due to activation of other pathways.
安尼可®(Penpulimab Injection)
Product information stated by NMPA in Approved Drug Catalog of China:
[Name]
Generic Name:Penpulimab Injection
Trade Name:安尼可®
English Name:Penpulimab Injection
Chinese Pinyin:Pai’anpuli Dankang Zhusheye
[Ingredients]
APIs:Penpulimab, prepared from Chinese hamster ovary cells by DNA recombination.
Excipients:Sodium acetate trihydrate, glacial acetic acid, sorbitol, and polysorbate 80.
[Appearance]
This product is colorless to pale yellow clear liquid, and may be slightly opalescent.
[Indications]
This product is indicated for adult patients with relapsed or refractory classical Hodgkin's lymphoma who have progressed on at least second-line systemic chemotherapy. Conditional approval of the indication is based on objective response rates and response duration from a single-arm clinical trial. Full approval for this indication will depend on the confirmation of a significant clinical benefit of Penpulimab over standard treatment in planned confirmatory randomized controlled clinical trials.
This product in combination with paclitaxel and carboplatin, is indicated for the first-line treatment of adult patients with metastatic non-squamous, non-small cell lung cancer (NSCLC).
[Specification]
100mg (10mL)/bottle
See the Directions for Use for [Usage and Dosage], [Adverse Reactions], [Contraindications], [Precautions], etc.
Indications
Hodgkin’s lymphoma
Lung cancer
Nasopharyngeal carcinoma
Liver cancer
Head and neck squamous cell carcinoma
Other tumors
·Penpulimab for treatment of patients with r/r cHL after at least second-line systemic chemotherapy treatment (Approved for marketing)
-
- 89.4%
ORR
- 47.1%
CR
-
72.1%PFS
(12 months)
-
100%OS
(12 months)
No Grade 3 or higher immune-related adverse events (irAEs) occurred
Hodgkin’s lymphoma is a malignant tumor that affects the lymph nodes and lymphatic system. Classical Hodgkin’s lymphoma (cHL) is the most common type, accounting for about 95% of cases. Hodgkin’s lymphoma has a recurrence rate of 10–30% after first-line treatment and about 5–10% of patients have primary refractory diseases.
·Penpulimab as first-line treatment for squamous NSCLC (NDA submitted)
- 69.7%
ORR
- 7 months
mPFS
There were no significant differences in the incidence of AEs and serious AEs compared with chemotherapy alone
Lung cancer is a malignant tumor with the highest mortality rate and the second highest morbidity in the world. Non-small cell lung cancer (NSCLC) accounts for approximately 80–85% of all lung cancer cases.
·Penpulimab in combination therapy as first-line treatment for nasopharyngeal carcinoma (Approved for marketing)
·Penpulimab as third-line treatment for nasopharyngeal carcinoma (Approved for marketing)
- 29.7%
ORR
- 3.65
monthsmPFS
- 18.63
monthsmOS
There are about 130,000 new cases of nasopharyngeal carcinoma (NPC) worldwide each year, about half of which occur in China. Recurrent or metastatic NPC has very limited treatment options and a poor prognosis.
·Penpulimab plus Anlotinib as first-line treatment for HCC
- 31.0%
ORR
- 82.8%
DCR
- 8.8
monthsmPFS
Liver cancer is the third leading cause of cancer death. In 2020, 410,000 cases of liver cancer occurred in China, with 390,000 deaths. China accounts for about 50% of liver cancer cases worldwide. Hepatocellular carcinoma (HCC) is the most major pathological type.
·Penpulimab plus Anlotinib in the treatment of R/M HNSCC
-
- 34.21%
ORR
- 76.32%
DCR
-
8.35
monthsmPFS
-
62.5%PFS
(6 months)
OS was not reached
Head and neck squamous cell carcinoma (HNSCC) is one of the most common malignancies. Globally, more than 700,000 new cases are diagnosed each year. More than 60% of HNSCC patients are locally advanced or advanced at initial diagnosis, and more than 50% of locally advanced HNSCC cases develop recurrence or metastasis within 3 years.
·Extensive clinical studies have been carried out on Penpulimab in the treatment of gastric cancer, head and neck tumors, hematomas and etc..













